With a population of approximately 10 million and a growing healthcare sector, Azerbaijan has seen a steady increase in demand for high-quality medicines and healthcare products.

Pharmaceutical registrations can be challenging for international regulatory affairs managers who are unfamiliar with the country’s specific requirements and procedures. To successfully register and market pharmaceutical products in Azerbaijan, it is crucial to have a thorough understanding of the regulatory framework, key authorities, and the step-by-step registration process.
We hope to provide international regulatory affairs managers with the essential information and insights needed to confidently approach the Azerbaijani market and streamline their product registration efforts.
By the end of this guide, readers will have a clear understanding of:
- The main regulatory authorities and their roles in pharmaceutical registration
- The legal framework governing medicines in Azerbaijan
- The types of pharmaceutical products subject to registration
- The standard and accelerated registration procedures and their requirements
- Post-registration obligations and pharmacovigilance requirements
- Labeling, packaging, and import/export regulations
- Recommendations for successful market entry and compliance
Regulatory Authorities and Legal Framework in Azerbaijan
Ministry of Health of the Republic of Azerbaijan
The Ministry of Health of the Republic of Azerbaijan is the primary regulatory authority responsible for overseeing the healthcare sector, including the regulation of pharmaceutical products. The Ministry’s key responsibilities include:
- Developing and implementing national policies and strategies related to healthcare and pharmaceuticals
- Issuing licenses for pharmaceutical activities, such as manufacturing, importing, and wholesaling
- Overseeing the registration, quality control, and safety monitoring of medicines
- Coordinating with other government agencies and international organizations on healthcare matters
The Ministry of Health comprises several departments and subordinate bodies that play specific roles in pharmaceutical regulation. The most relevant departments for international regulatory affairs managers include:
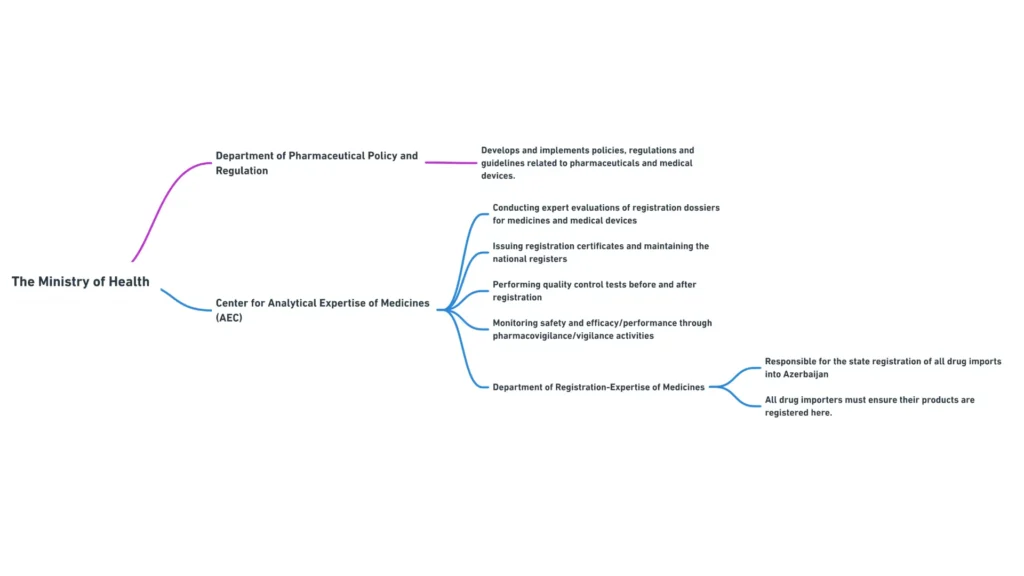
- Department of Pharmaceutical Policy and Regulation: Responsible for developing and implementing pharmaceutical policies, regulations, and guidelines
- Department of Licensing and Certification: Handles the licensing of pharmaceutical activities and the certification of pharmaceutical professionals
- Department of International Relations: Coordinates the Ministry’s cooperation with international organizations and foreign regulatory authorities
Azerbaijan MOH and Departments Handling Pharmaceuticals
Center for Analytical Expertise of Medicines
The Center for Analytical Expertise of Medicines is a subordinate body of the Ministry of Health that plays a crucial role in the registration and quality control of pharmaceutical products in Azerbaijan. The Center’s main functions include:
- Conducting expert evaluations of registration dossiers for medicines
- Issuing registration certificates and maintaining the national register of medicines
- Performing quality control tests on pharmaceutical products before and after registration
- Monitoring the safety and efficacy of registered medicines through pharmacovigilance activities
The Center for Analytical Expertise of Medicines is the primary point of contact for international regulatory affairs managers during the registration process. It is essential to maintain clear communication with the Center and promptly address any queries or requests for additional information to ensure a smooth registration process.
Relevant Legislation and Regulations
The legal framework governing pharmaceuticals in Azerbaijan consists of laws, decrees, and regulations that set out the requirements and procedures for the registration, manufacture, import, and distribution of medicines.
The most important legislation for international regulatory affairs managers to be aware of includes:
- Law on Medicines (2006): The primary law regulating pharmaceutical products in Azerbaijan, which covers aspects such as registration, manufacturing, quality control, and pharmacovigilance. The law has undergone several amendments since its adoption to align with international standards and best practices.
- Decree No. 108 on the Rules for State Registration of Medicines (2007): Outlines the detailed requirements and procedures for the registration of pharmaceutical products, including the content of registration dossiers, timelines, and fees.
- Decree No. 137 on the Rules for Conducting Expertise of Medicines (2007): Specifies the procedures and criteria for the expert evaluation of registration dossiers by the Center for Analytical Expertise of Medicines.
- Resolution No. 137 on the Rules for Importing and Exporting Medicines (2007): Sets out the requirements and procedures for importing and exporting pharmaceutical products, including licensing, customs clearance, and documentation.
In addition to these key pieces of legislation, international regulatory affairs managers should also stay informed about any amendments, supplementary regulations, or guidance documents issued by the Ministry of Health or the Center for Analytical Expertise of Medicines.
Regularly consulting the official websites and newsletters of these authorities can help ensure compliance with the most up-to-date requirements.
Types of Pharmaceutical Products Subject to Registration
In Azerbaijan, most pharmaceutical products must be registered with the Center for Analytical Expertise of Medicines before they can be marketed and sold. The registration requirement applies to both imported and domestically manufactured products.
The main types of pharmaceutical products subject to registration include:
- Original medicines: Innovative pharmaceutical products containing new active substances or new combinations of active substances that have not been previously registered in Azerbaijan.
- Generic medicines: Pharmaceutical products that contain the same active substances, dosage form, and strength as an already registered original medicine and are intended to be interchangeable with the original product.
- Combination products: Medicines that contain two or more active substances in a fixed-dose combination, where each active substance contributes to the overall therapeutic effect.
- Pharmaceutical forms and dosages: Different pharmaceutical forms (e.g., tablets, capsules, syrups) and dosages of the same active substance require separate registration, even if they are produced by the same manufacturer.
However, certain types of pharmaceutical products may be exempt from the registration requirement, such as:
- Medicines prepared in pharmacies according to a doctor’s prescription for individual patients
- Medicines imported for personal use in quantities not exceeding a specified limit
- Medicines imported for clinical trials or scientific research purposes, with prior approval from the Ministry of Health
It is essential for international regulatory affairs managers to carefully assess their product portfolio and determine which products require registration in Azerbaijan. This assessment should take into account factors such as the composition, intended use, and potential market demand for each product.
| Type of Product | Registration Requirement |
| Original medicines | Yes |
| Generic medicines | Yes |
| Combination products | Yes |
| Different pharmaceutical forms and dosages | Separate registration required |
| Medicines prepared in pharmacies for individual patients | Exempt |
| Medicines imported for personal use (limited quantities) | Exempt |
| Medicines imported for clinical trials or research (with approval) | Exempt |
Pharmaceutical Registration Procedures and Timelines in Azerbaijan
To register a pharmaceutical product in Azerbaijan, international regulatory affairs managers must follow the appropriate registration procedure and submit a comprehensive dossier to the Center for Analytical Expertise of Medicines.
The registration process consists of two main procedures: the standard registration procedure and the accelerated registration procedure.
Standard Pharmaceutical Registration Procedure in Azerbaijan
The standard registration procedure is the most common pathway for registering pharmaceutical products in Azerbaijan. This procedure involves a thorough evaluation of the product’s quality, safety, and efficacy by the Center for Analytical Expertise of Medicines.
The key steps and requirements of the standard registration procedure are as follows:
- Dossier Submission: The applicant must submit a complete registration dossier to the Center for Analytical Expertise of Medicines. The dossier should be prepared in accordance with the Common Technical Document (CTD) format and include the following modules:
- Administrative information (Module 1)
- Quality data (Module 2)
- Non-clinical data (Module 3)
- Clinical data (Module 4)
- Pharmacovigilance and risk management plan (Module 5)
- The dossier must be submitted in both hard copy and electronic format, along with the required registration fee.
- Preliminary Expertise: Upon receiving the dossier, the Center for Analytical Expertise of Medicines conducts a preliminary review to ensure that the application is complete and meets the formal requirements. If any deficiencies are identified, the applicant will be notified and given a specified timeframe to address them.
- Specialized Expertise: Once the dossier passes the preliminary review, it undergoes a specialized expertise, which involves a detailed evaluation of the product’s quality, safety, and efficacy data by a panel of experts. This stage may include:
- Assessment of the pharmaceutical and analytical documentation
- Review of non-clinical and clinical study reports
- Evaluation of the product’s benefit-risk profile
- Inspection of the manufacturing site(s) for compliance with Good Manufacturing Practices (GMP)
- During this stage, the Center for Analytical Expertise of Medicines may request additional information or clarification from the applicant. It is crucial to respond promptly and thoroughly to these requests to avoid delays in the registration process.
- Decision and Certificate Issuance: Based on the outcome of the specialized expertise, the Center for Analytical Expertise of Medicines makes a decision on whether to approve or reject the registration application. If approved, the applicant will receive a registration certificate valid for five years. The registered product will also be included in the national register of medicines.
The standard registration procedure typically takes between 6 to 12 months from the date of dossier submission to the issuance of the registration certificate.
However, this timeline may vary depending on the complexity of the product, the quality of the submitted dossier, and the workload of the Center for Analytical Expertise of Medicines.
Accelerated Registration Procedure in Azerbaijan
In July 2020, Azerbaijan introduced an accelerated registration procedure to streamline the registration process for certain categories of pharmaceutical products. This procedure aims to reduce the registration timeline while still ensuring the quality, safety, and efficacy of the products.
To be eligible for the accelerated registration procedure, a pharmaceutical product must meet one of the following criteria:
- The product is already registered in countries with stringent regulatory authorities (e.g., EU, USA, Japan, Australia, Canada)
- The product is prequalified by the World Health Organization (WHO)
- The product is intended to treat rare diseases or conditions with high unmet medical needs
Under the accelerated registration procedure, the Center for Analytical Expertise of Medicines will prioritize the evaluation of eligible products and aim to complete the registration process within 3 to 6 months.
However, the applicant must still submit a complete registration dossier and promptly address any queries or requests for additional information from the authorities.
It is important to note that the accelerated registration procedure does not exempt products from the requirement to demonstrate quality, safety, and efficacy. The same scientific and technical standards apply as in the standard registration procedure.
Refusal of Pharmaceutical Registration in Azerbaijan
In some cases, the Center for Analytical Expertise of Medicines may refuse to register a pharmaceutical product. The most common grounds for refusal include:
- Insufficient or inadequate data to demonstrate the product’s quality, safety, or efficacy
- Non-compliance with Good Manufacturing Practices (GMP) or other relevant standards
- Misrepresentation or falsification of information in the registration dossier
- Failure to address deficiencies or provide additional information requested by the authorities within the specified timeframe
If a registration application is refused, the applicant will receive a written notification outlining the reasons for the refusal. The applicant may appeal the decision within 30 days by submitting a written request to the Ministry of Health.
The appeal will be reviewed by a higher-level expert committee, which will make a final decision on the registration application.
Changes to Registered Pharmaceuticals
After a pharmaceutical product has been registered in Azerbaijan, any changes to the product’s composition, manufacturing process, labeling, or other aspects must be reported to the Center for Analytical Expertise of Medicines.
The type of change will determine whether a notification or a re-registration is required.
Types of Changes Requiring Notification or Re-registration
- Composition and Manufacturing Changes:
- Minor changes to the composition or manufacturing process that do not affect the product’s quality, safety, or efficacy may require only a notification to the authorities.
- Major changes, such as the addition or removal of an active substance, a significant change in the manufacturing process, or a change in the manufacturing site, may require re-registration.
- Labeling and Packaging Modifications:
- Minor changes to the labeling or packaging, such as updates to the manufacturer’s contact information or the addition of a barcode, may require only a notification.
- Major changes, such as modifications to the product’s name, indication, dosage, or storage conditions, may require re-registration.
- Indications and Contraindications:
- Any changes to the product’s approved indications, contraindications, or other aspects of the prescribing information will require re-registration.
Procedure for Submitting Regulatory Variation Change Requests in Azerbaijan
To notify the Center for Analytical Expertise of Medicines of a change or to request re-registration, the marketing authorization holder must submit a written application along with supporting documentation.
The application should clearly describe the nature and scope of the change and provide evidence to demonstrate that the change does not adversely affect the product’s quality, safety, or efficacy.
The Center for Analytical Expertise of Medicines will review the change request and make a decision on whether to approve the change or require additional information. The timeline for the review process varies depending on the complexity of the change and the workload of the authorities.
| Type of Change | Notification | Re-registration |
| Minor composition or manufacturing changes | ✓ | |
| Major composition or manufacturing changes | ✓ | |
| Minor labeling or packaging modifications | ✓ | |
| Major labeling or packaging modifications | ✓ | |
| Changes to indications or contraindications | ✓ |
Failure to comply with the change notification or re-registration requirements may result in penalties or the suspension or revocation of the product’s registration.
Renewal and Cancellation of Pharmaceutical Registration
In Azerbaijan, the registration of a pharmaceutical product is valid for five years from the date of issuance of the registration certificate. To continue marketing the product after the expiration of the registration, the marketing authorization holder must apply for renewal.
Renewal Application Process and Requirements in Azerbaijan
The renewal application must be submitted to the Center for Analytical Expertise of Medicines at least 90 days before the expiration of the current registration.
The application should include the following documents:
- A completed renewal application form
- A copy of the current registration certificate
- Updated quality, safety, and efficacy data, including:
- Periodic safety update reports (PSURs)
- Post-marketing surveillance data
- Any new clinical or non-clinical studies conducted since the initial registration
- A declaration confirming that the product’s composition, manufacturing process, and other aspects remain unchanged or detailing any changes made since the initial registration
- Samples of the product and reference standards for quality control testing, if requested by the authorities
The Center for Analytical Expertise of Medicines will review the renewal application and make a decision on whether to grant the renewal within 60 days. If the authorities require additional information or clarification, the review timeline may be extended.
Grounds for Cancellation of Pharmaceutical Registration
The Center for Analytical Expertise of Medicines may cancel the registration of a pharmaceutical product in the following circumstances:
- The marketing authorization holder fails to apply for renewal before the expiration of the current registration
- The product is found to be unsafe, ineffective, or of substandard quality based on post-marketing surveillance data or other evidence
- The marketing authorization holder fails to comply with pharmacovigilance obligations or other post-registration requirements
- The product is not marketed in Azerbaijan for three consecutive years without a valid reason
- The marketing authorization holder requests the cancellation of the registration
If the Center for Analytical Expertise of Medicines intends to cancel a product’s registration, it will notify the marketing authorization holder in writing, outlining the reasons for the proposed cancellation.
The marketing authorization holder has the right to appeal the decision within 30 days by submitting a written request to the Ministry of Health.
Consequences of Non-Renewal or Cancellation
If a pharmaceutical product’s registration is not renewed or is cancelled, the marketing authorization holder must:
- Cease all marketing and distribution activities for the product in Azerbaijan
- Withdraw the product from the market within the timeframe specified by the authorities
- Notify healthcare professionals and other relevant stakeholders about the non-renewal or cancellation
- Cooperate with the authorities in any recall or other risk mitigation measures deemed necessary to protect public health
Failure to comply with these obligations may result in penalties, including fines and legal action.
Pharmacovigilance and Post-Marketing Obligations
To ensure the ongoing safety and efficacy of registered pharmaceutical products, marketing authorization holders in Azerbaijan must comply with pharmacovigilance and other post-marketing obligations.
Pharmacovigilance System Requirements in Azerbaijan
Marketing authorization holders must establish and maintain a pharmacovigilance system that complies with the requirements set out in the Law on Medicines and relevant regulations.
The key elements of a pharmacovigilance system include:
- Appointment of a Local Contact Person: The marketing authorization holder must appoint a qualified person responsible for pharmacovigilance (QPPV) who resides in Azerbaijan and serves as the primary contact for the authorities on pharmacovigilance matters.
- Adverse Event Reporting: The marketing authorization holder must report all serious adverse events associated with the use of its products in Azerbaijan to the Center for Analytical Expertise of Medicines within 15 days of receiving the information. Non-serious adverse events must be reported within 90 days.
- Signal Detection and Risk Management: The marketing authorization holder must continuously monitor the safety profile of its products and identify any new risks or changes in the benefit-risk balance. If a new safety signal is detected, the marketing authorization holder must notify the authorities and implement appropriate risk management measures.
Periodic Safety Update Reports (PSURs)
Marketing authorization holders must submit periodic safety update reports (PSURs) to the Center for Analytical Expertise of Medicines according to the following schedule:
- Every 6 months for the first 2 years after registration
- Annually for the next 2 years
- Every 3 years thereafter
PSURs should provide a comprehensive overview of the product’s safety profile, including an analysis of all adverse events reported worldwide, any new safety signals identified, and any changes in the benefit-risk balance.
Inspections and Audits of Marketing Authorization Holders
The Center for Analytical Expertise of Medicines may conduct inspections and audits of marketing authorization holders to ensure compliance with pharmacovigilance and other post-marketing obligations.
During an inspection or audit, the authorities may review the marketing authorization holder’s pharmacovigilance system, adverse event reporting processes, risk management plans, and other relevant documentation.
Marketing authorization holders must cooperate fully with the authorities during inspections and audits and promptly address any deficiencies or non-compliance identified.
Penalties for Non-Compliance
Failure to comply with pharmacovigilance and other post-marketing obligations may result in penalties, including:
- Fines and administrative sanctions
- Suspension or revocation of the product’s registration
- Criminal prosecution in cases of serious non-compliance or fraud
To avoid these consequences, international regulatory affairs managers must ensure that their companies have robust pharmacovigilance systems in place and regularly review and update their processes to maintain compliance with Azerbaijani requirements.
Labeling and Packaging Requirements in Azerbaijan
Pharmaceutical products marketed in Azerbaijan must comply with specific labeling and packaging requirements to ensure that healthcare professionals and patients have access to accurate and complete information about the product.
Language and Content Requirements
All labeling and packaging materials for pharmaceutical products must be in the Azerbaijani language.
In addition to the product name and the name and address of the manufacturer, the following information must be included on the label and package insert:
- Composition, including active substances and excipients
- Pharmaceutical form and strength
- Indications and contraindications
- Dosage and method of administration
- Warnings and precautions
- Adverse reactions
- Storage conditions and shelf life
- Batch number and expiry date
- Registration number and date of registration
Specific Requirements for Controlled Substances and Prescription Medicines
Pharmaceutical products containing controlled substances or requiring a prescription must include additional labeling elements, such as:
- The international non-proprietary name (INN) of the controlled substance
- The concentration of the controlled substance
- The prescription status of the product (e.g., “Prescription only”)
- Special storage and handling requirements
Approval Process for Labeling and Packaging Materials
Before a pharmaceutical product can be marketed in Azerbaijan, the labeling and packaging materials must be approved by the Center for Analytical Expertise of Medicines. The marketing authorization holder must submit samples of the proposed labeling and packaging along with the registration dossier or as part of a post-registration change request.
The Center for Analytical Expertise of Medicines will review the labeling and packaging materials to ensure that they comply with the language and content requirements and accurately reflect the product’s approved prescribing information.
If the authorities require changes to the labeling or packaging, the marketing authorization holder must revise the materials and resubmit them for approval.
Import and Export Regulations
International regulatory affairs managers must also be aware of the import and export regulations governing pharmaceutical products in Azerbaijan.
Licensing Requirements for Pharmaceutical Importers and Exporters
Companies engaged in the import or export of pharmaceutical products in Azerbaijan must obtain the appropriate licenses from the Ministry of Health.
The key licensing requirements include:
- Importer License: Companies that import pharmaceutical products into Azerbaijan must obtain an importer license. To be eligible for an importer license, the company must have:
- A registered legal entity in Azerbaijan
- Suitable premises and equipment for the storage and handling of pharmaceutical products
- Qualified personnel, including a responsible pharmacist
- A quality control system that complies with Good Distribution Practices (GDP)
- Exporter License: Companies that export pharmaceutical products from Azerbaijan must obtain an exporter license. To be eligible for an exporter license, the company must have:
- A registered legal entity in Azerbaijan
- A valid manufacturing license or a contract with a licensed manufacturer
- Suitable premises and equipment for the storage and handling of pharmaceutical products
- Qualified personnel, including a responsible pharmacist
- A quality control system that complies with Good Manufacturing Practices (GMP) and Good Distribution Practices (GDP)
Customs Clearance Procedures and Documentation
When importing or exporting pharmaceutical products, companies must comply with the customs clearance procedures and provide the necessary documentation, including:
- Commercial invoice
- Packing list
- Certificate of analysis
- Certificate of origin
- Registration certificate or import permit, as applicable
- Other documents required by the customs authorities
Special Considerations for Controlled Substances and Cold Chain Products
Pharmaceutical products containing controlled substances or requiring cold chain storage and transportation are subject to additional import and export requirements. For controlled substances, companies must obtain an import or export permit from the Ministry of Health for each shipment.
For cold chain products, companies must ensure that the products are stored and transported under the required temperature conditions and provide evidence of temperature monitoring during customs clearance.
Final Thoughts on Registering Pharmaceuticals in Azerbaijan
To successfully complete the Azerbaijani pharmaceutical procedures, international regulatory affairs managers should consider the following recommendations:
- Engage Local Regulatory Experts and Partners: Working with local regulatory experts and partners, such as Delta Medical, can help international companies understand and comply with Azerbaijani requirements, avoid common pitfalls, and accelerate the registration process.
- Plan Ahead and Allow Sufficient Time for Registration: The registration process in Azerbaijan can be lengthy and complex, particularly for innovative products. International companies should plan ahead and allow sufficient time for dossier preparation, submission, and review to avoid delays in market entry.
- Prioritize Products with High Market Potential: To maximize the return on investment, international companies should prioritize the registration of products with high market potential in Azerbaijan, considering factors such as disease prevalence, unmet medical needs, and competition.
- Ensure Compliance with Post-Registration Requirements: International companies must establish robust pharmacovigilance systems and processes to comply with post-registration requirements in Azerbaijan. Regularly reviewing and updating these systems can help avoid penalties and maintain the smooth operation of the business.
- Stay Informed of Regulatory Changes and Updates: The Azerbaijani pharmaceutical regulatory landscape is constantly evolving, with new regulations and guidelines being introduced periodically. International regulatory affairs managers should stay informed of these changes and adapt their strategies and processes accordingly.
By following these recommendations and working closely with local partners, international companies can successfully navigate the Azerbaijani pharmaceutical market and contribute to improving patient access to safe, effective, and high-quality medicines.


